Today, Ms Chia brought us to the lab, to carry out 2 experiments. These 2 experiments were to determine densities.
The first experiment was to find the density of a glass marble. Firstly, we must find the mass of the marbles.Then, we must find the diameter of one marble, so we can find its volume. As the diameter of the marble is very small, we need the help of the micrometer screw gauge.
Finally, to find the density of the marble, we must take its mass divided by its volume.
Ms Chia wanted us to use 5 marbles instead of 1 as that can help to minimize any errors resulting from inconsistencies of the marbles :D
The second experiment was to determine the density of an irregular solid. In this experiment, we used a glass stopper.
Firstly, we must weigh the mass of it. Afterwards, we can lower it into a cylinder of water. We were told to observe the increase in the water level. The increase was the volume of the glass stopper. To prevent the water from splashing out, we attached a string to it so we could lower it slowly into the water.
Lastly, to find its density, we had to take its mass divided by its volume.
1P8: Brownian Motion
Today, Ms Chia did not bring us to the Science lab for the practical. Instead, it was carried out in our classrooms. Basically, she showed us a video on Brownian motion and wanted us to observe the movement of the smoke particles as shown in the video.
The smoke particles moved in random directions and is in a continuous motion.
This practical was very useful as it helped me understand the Brownian motion theory better :D
1P9: Investigating Mixtures and Compounds
Today, Ms Chia brought us to the lab to investigate the properties of a mixture and a compound. We were allowed to use sulphur powder and iron filings.
For the first experiment, we must describe the appearance of the sulphur powder. Afterwards, we were given a magnet and moved it close to the sulphur powder. As sulphur is not magnetic, it was not attracted to the magnet.

We repeated the experiment again on iron filing. As iron is a magnetic material, it was attracted to the magnet.
The second experiment involved a mixture of sulphur powder and iron filings. We must jot down its appearance and again, use a magnet and move it close to it. As a result, only the dark grey-coloured iron filings got attracted to the magnet. The experiment is illustrated in the picture below:
The last experiment was heating the mixture of sulphur and iron over a Bunsen flame. We were told to heat it until no more change occurs, then describe its appearance.
The mixture became a black solid (iron sulphide). When the magnet was brought close to the compound, it did not get attracted. Hence, I can conclude that iron sulphide is not magnetic.
1P10: Forming Compounds
Yippee! Ms Chia brought us to the Science lab again to do experiments. Today, we did 3 cool experiments!
The first experiment involved Magnesium. When Ms Chia heated it over a Bunsen flame, it actually gave off a cool, bright white dazzling light! It was real cool as I had never seen such things before. Yeah, I know you would not know how excited we were when it was heated. Maybe the photo below might help you:
After burning for about 2 minutes, white ash was formed. From this, we can deduce that Magnesium + Oxygen becomes magnesium oxide.
The second experiment we did was using an element to react with a compound. We were told to add dilute sulphuric acid to half a spatula of iron filings. An effervesence of a colourless and odourless gas was formed and the solution turned warm. Also, the iron filings changed from grey to light grey.
Here is the word equation: Iron + Sulphur --- iron sulphate + hydrogen gas.
(ignore the test tube on your right)
The last experiment we did was using 2 compounds to get a reaction. We were instructed to add lead(II) nitrate to sodium choloride solution. When we did so, a white precipitate was formed together with a colourless solution. The word equation is as follows:
Sodium Chloride + lead(II) nitrate --- sodium nitrate + lead(II) chloride.
1P11: Which can dissolve more?
Today, Ms Chia brought us to the lab to find out the different solubilities of certain solutes. The solutes we tested today includes: Salt, Baking soda and iodine crystals. We were supposed to keep adding the solute into the solvent (water), until the solute cannot be dissolved any more.
After the experiment, we found out that salt is the most soluble in water, whereas iodine is the least soluble.
1P12: A Separation Problem
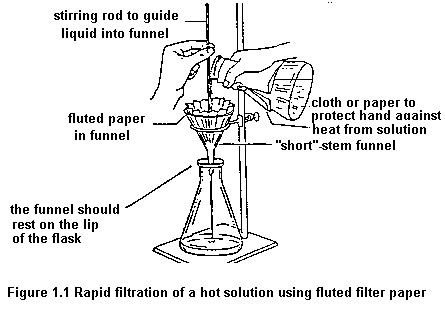
This is sad... but it is our last lab session for term 2! Anyway, today, we were supposed to separate a mixture of ingredients. I consists of iron filings, small Styrofoam beads, salt and sand. We had to use physical methods to separate them.
Firstly, we used a magnet to remove the iron filings. Afterwards, we fill the remaining substance with mixture so that the Styrofoam beads would float to the surface. Then, we would filter the remaining substance until all the salt water get filtered through, leaving the sand behind on the filter paper. Lastly, we would boil the salt water until all the water has evaporated, leaving the salt behind as residue.







No comments:
Post a Comment